Purpose: The introduction of targeted agents has been a major advance in the treatment of classical Hodgkin lymphoma (HL) but the optimal application and sequencing of these agents is unclear. The CD30-targeted antibody-drug conjugate brentuximab vedotin (BV) is licensed for treatment of relapsed and refractory (R/R) HL as monotherapy and is available in the United Kingdom as third-line (3L) therapy for HL. BV has been evaluated in combination with chemotherapy in single-arm trials with very promising results, but randomised data are lacking. The aim of this analysis was to compare the efficacy of BV monotherapy with BV plus bendamustine (BVB) as 3L treatment of HL in a real-world UK cohort.
Methods: Data were retrospectively collectively for consecutive patients receiving treatment for R/R HL with intent to consolidate with stem cell transplant (SCT) between 01/2015 and 12/2019 at 11 UK centres. Use of BVB was principally determined by availability, due to regional differences in BV funding in the UK, rather than patient disposition. Response was assessed by PET according to the Lugano Classification. Most analyses use descriptive statistics. Time-to-event analyses are measured from the start of 3L treatment until the first event (death for overall survival and death or further therapy for treatment failure) with groups compared using Kaplan Meier survival analysis and Cox regression.
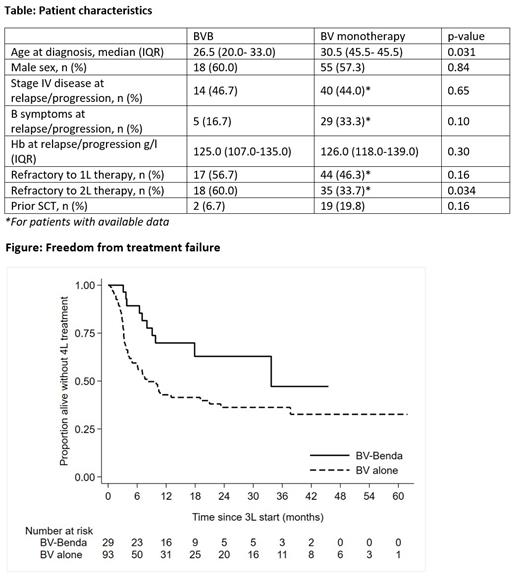
Results: 96 patients received BV and 30 received BVB. Baseline demographics are provided in the Table. Patients receiving BVB were slightly younger in age at diagnosis (p=0.031), but a slightly higher proportion had primary refractory disease (p=0.16) and failed to respond to second-line therapy (p=0.034). Numerically fewer patients in the BVB arm had received prior SCT (p=0.16).
Patients received a median of 4 cycles of BV monotherapy (range 3 - 5). Patients treated with BVB received a median of 3 cycles of bendamustine (IQR 2 - 4) and 4 cycles of BV (IQR 3 - 5); 2 patients discontinued bendamustine after 1 cycle due to adverse reactions.
Complete metabolic response was achieved in 34 patients (37.4%) with BV and 22 patients (73.3%) with BVB, with overall response rates of 57.3% and 93.3%, respectively (OR 9.2; 95% CI: 2.06 - 40.86; p=0.004). SCT consolidation was performed after 3L treatment in 33 patients (34.4%) following BV (26 autologous, 7 allogeneic) and 22 patients (73.3%) after BVB (17 autologous, 5 allogeneic).
Median follow-up was 30.1 months after BV (IQR: 15.3 - 46.8) and 18.1 months after BVB (15.4 - 29.8). Freedom-from-treatment-failure (FFTF) rates at 18 months were 41.5% (95% CI: 30.8 - 51.8) after BV and 62.9% (39.3 - 79.4) after BVB (HR 0.45, 95% CI 0.23 - 0.88; p=0.019; see Figure). Overall survival rates at 18 months were 76.9% (66.0 - 84.7) after BV and 88.4% (68.1 - 96.1) after BVB (HR 0.37, 95% CI 11 - 1.23; p=0.11. There were 29 deaths following BV, including 17 (17.7%) due to HL, 6 (6.3%) due to toxicity of SCT or subsequent treatment and 6 (6.3%) due to other causes/unknown. There were 3 deaths following BVB, all due to toxicity of SCT or subsequent treatment (10%).
Eight patients received single-agent bendamustine after failure of BV monotherapy, at a median of 2.4 months after commencing BV. The median number of bendamustine cycles delivered was 2 (range 1 - 6). The overall response rates was 62.5%. Two patients (25%) achieved complete metabolic response; both underwent subsequent SCT.
Conclusion: In this retrospective analysis, use of BVB as 3L treatment for R/R HL was associated with higher response rates, transplant rates and FFTF compared with BV monotherapy. Although toxicity data were not available, our results are otherwise consistent with phase 2 trial data with BVB as second-line therapy (LaCasce, Blood 2018;132:40, Broccoli, Blood Cancer Journal 2019;9:100). These data demonstrate that BVB is a highly effective regimen in R/R HL, and support its use in transplant-fit patients.
Disclosures
Shotton:Servier: Honoraria, Other: Travel to scientific conferences; Beigene: Other: Travel to scientific conferences. Kirkwood:Kite-Gilead: Honoraria. Ferguson:Abbvie: Other: Conference fees. Martinez-Calle:Abbvie: Honoraria, Other: Conference sponsorship; Beigene: Honoraria; AstraZeneca: Honoraria, Other: Conference sponsorship; Takeda: Honoraria. Gallop-Evans:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Educational support. McKay:Gilead/Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel to scientific conferences; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel to scientific conferences; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel to scientific conferences; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees. Gohil:UCLB: Patents & Royalties; Novalgen: Consultancy; Freeline Therapeutics: Consultancy; Janssen: Speakers Bureau; Abbvie: Honoraria, Other: Travel; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria, Speakers Bureau. Collins:Amgen: Research Funding; Pfizer: Research Funding; BMS: Research Funding; Beigene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Astra Zeneca: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Speakers Bureau; Daiichi Sankyo: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau. Phillips:Takeda: Honoraria, Research Funding; Gilead: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal